Professor Dario Braga has a long standing experience in patent litigations over crystal forms. Based on the experience in solid state techniques and on the thermodynamic and kinetic reasons for the appearance, existence, relative stability of crystal forms and the relationship between structure and crystal modification and physico-chemical properties. Dario Braga has provided technical opinions (also with deposition in the court) on litigations concerning a series of drug patents (es. levofolinic acid, lenvatininb mesylate, calcipotriol monohydrate, azozystrobin, eszopiclone, moxifloxacin hydrochloride and rifaximin). Contact by email dario.braga@unibo.it
The relevance of crystal forms in the pharma world
It is now clear that the crystallization of an active pharmaceutical ingredient (API) obtained after long, extenuating and expensive studies, is not, simply, the solid form of a new pharmaceutical but, rather, the beginning of another long journey in the quest for crystalline materials with stable and controllable properties.
This journey requires specific skill and training and the ability to understand the results of a variety of solid-state methods and techniques used in combination. The goal is that of minimizing, if not eliminating, the chances of an unexpected appearance of an unknown new crystal form of a drug at later stages of its development, if not its marketing.
And this is not all. It is not only necessary to explore as thoroughly as possible the “crystal space domain” of the molecule of interest but also to be able to follow the production, storage and distribution of the product to guarantee persistence of the solid form, hence of the selected properties.
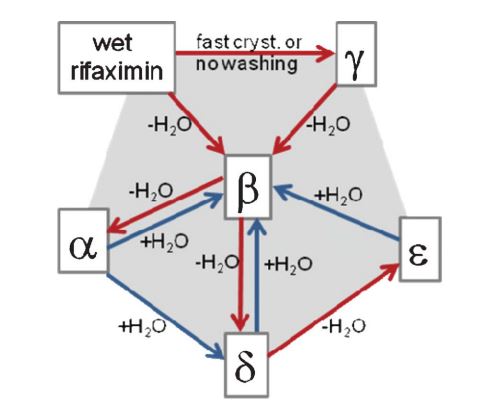
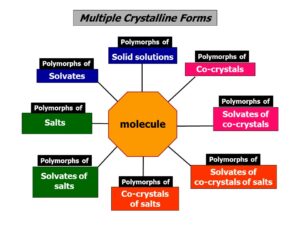 Crystal forms discovery and assessment, whether single crystal polymorphs, hydrates, and more generally solvates, salts, co-crystals etc., has indeed become part of the system of quality control in the pharmaceutical industry. It is necessary to make sure not only that the scaling-up from laboratory preparation to industrial production does not introduce variations in crystal forms (persistence of properties) but also that all accessible crystal form variations of the API of interest have been taken into consideration (exclusion of alternatives).
Crystal forms discovery and assessment, whether single crystal polymorphs, hydrates, and more generally solvates, salts, co-crystals etc., has indeed become part of the system of quality control in the pharmaceutical industry. It is necessary to make sure not only that the scaling-up from laboratory preparation to industrial production does not introduce variations in crystal forms (persistence of properties) but also that all accessible crystal form variations of the API of interest have been taken into consideration (exclusion of alternatives).
Crystal forms assessment also guarantees that the product conforms to the guidelines of the appropriate regulatory agencies and does not infringe the intellectual property protection that may cover other crystal forms.
Both initial polymorph screening and continuous crystal form assessment require the combined use of several solid-state techniques, among them (not exclusively or in any preferential order): microscopy and hot stage microscopy (HSM), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), infrared and Raman spectroscopy (IR and Raman), single crystal/powder X-ray diffraction (SCXD, PXD), solid state nuclear magnetic resonance spectroscopy (SSNMR).
Litigations over crystal forms as well as the impact on pharmaceutical industries of famous “incidents” (Norvir, Neupro) have fueled research in solid-state chemistry, and oriented the experience and competence of many research groups worldwide. Although the motivation of such studies arises essentially from safety and commercial necessities, the consequences are important also for fundamental science.
Since crystal modifications of a substance represent different crystal structures with potentially different properties, the discovery or preparation of a new crystal form represents an opportunity to claim an invention that might be patentable. A particular crystalline modification can possess considerable chemical, physical or biological advantages over its congeners, and the granting and maintenance of patent exclusivity over the rights to particular crystal form may have considerable economic consequences. As a result, it is not surprising that many patent litigations involve crystal forms, whether single molecule polymorphs, solvated and hydrates, and more recently, of co-crystals.
Although rules and regulations of patents differ from country to country there is some degree of standardization resulting from the World Trade Agreement and subsequent legislation in Europe, USA and various countries. For instance, in the United Kingdom, a new crystal modification is not prima facia patentable; the inventor must demonstrate that it is an unobvious variant of the previously known material.
The reader interested in leaning more about the theme of crystal forms selection, characterization and utilization as well as the im portant aspects of intellectual property protection is addressed to some of my publications as an entry point in the extremely vast and still expanding literature.