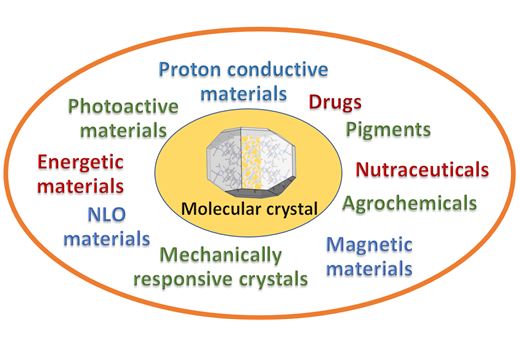
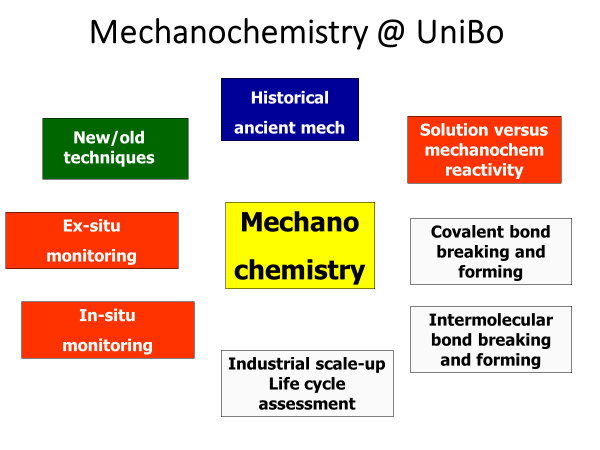
The course of Molecular Crystal Engineering at the University of Bologna
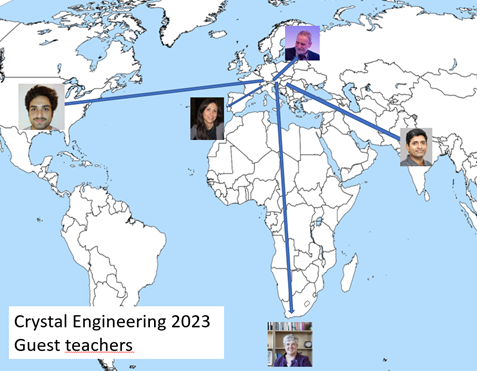
For the academic year 2023-2024 external contributions were provided by guest professors Luca Catalano (USA), Susan Bourne (South Africa), Vania Andrè (Portugal), Andrzej Katrusiak (Poland) and Malla Reddy Chilla (India).
1. Introduction. Hystorical background. When crystal engineering was born. Tutorials on most relevant techniques for the investigation of the solid state (IR and RAMAN spectroscopies, solid state NMR, X-ray diffraction, differential scanning calorimetry, TGA)
2. Intermolecular interactions in molecular crystals, amorphous substances, hydrogen bonds, halogen bonds, sigma-hole interactions, coordination bonds to construct superstructures.
3. Crystal forms. Multeplicity of crystal forms for a same substance, crystal polymorphism, hydrates, solvates, salts, co-crystals and their polymorphs
4. Crystal Polymorphism. The issue of identification, characterization, usage of crystal forms. The impact in the pharmaceutical field. Aspects related to intellectual property issues. Enantiotropic and monotropic polymorphism. Case studies.
5. Crystallization. Main techniques. From solution, from melt, The kinetic problem and the quest for the most thermodynamically stable form.
6. Solvates and hydrates. Dynamic vapour sorption. Solubility and stability of hydrates. The formation of solvates and hydrates via crystallization. Solvent removal and interconversion. Thermodynamic and calorimetric aspects. Case studies.The case of rifaximin.
7. Co-crystals. Preparation of multicomponent molecular crystals, the problem of acid-base crystals, proton transfer. mechanochemical prep. Aspects related to IP issues and patentability. The ionic co-crystals. Case studies.8. Chirality. Chiral crystals, relationship between chirality at the molecular level and at crystal level. Racemic mixtures, racemic conglomerates, racemates. The importance of chiral resolution. Case studies.
9. Metal Organic Frameworks. Hystorical background: the relationship between coordination chemistry and coordination networks: spacers and knots. Case studies. The properties of MOF. Their industrial utilization: gas storage, catalysis in nanocavities, molecular sieves. Adsorption and desadsorption of molecules in/from MOF.
10. HOFs. Organic hydrogen bonded networks. Preparation and applications as porous materials.