There is little doubt that the design, synthesis, characterization, and biological and pharmacological evaluation of new active ingredients are the first and paramount objectives of research in the pharmaceutical field.
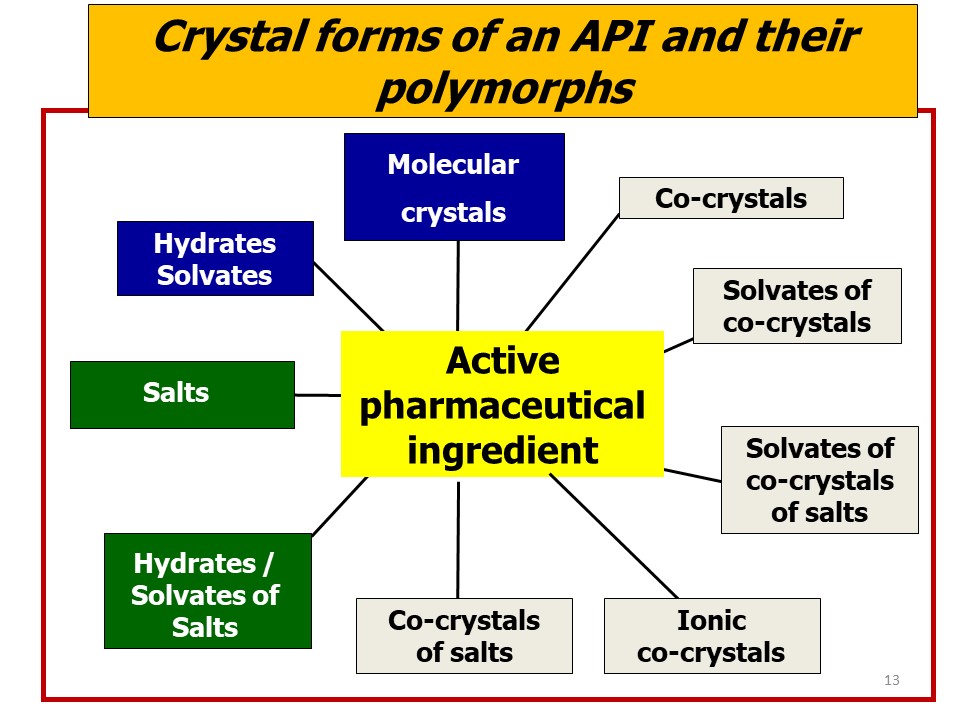
However, obtaining a new drug is not enough: the road from bench to market is often long, steep and tortuous. One aspect that has emerged in all its relevance is that of the impact of the solid-state form of the active ingredient on its utilization as a drug. The vast majority of drugs is constituted of organic molecules. Organic molecules are often structurally non-rigid and conformationally adaptable, and often carry functional groups that form strong and directional intermolecular interactions (hydrogen bonds, halogen bonds, etc.) or that confer anisotropy and polarity, hence impose orientational preference to surrounding molecules, whether of the same or of different type. This variability has consequences when the active ingredient is crystallized from solution, melt, or vapor phase, because the formation of a solid from a less condensed phase is the outcome of a competition between kinetics and thermodynamic aspects. The isolation of stable or metastable crystal forms, whether enantiotropically or monotropically related, is a manifestation of such dualism, as it is the precipitation of a solvate or of an unsolvated crystal of the API from solution. As we have seen in this review, an active ingredient can be brought in the solid state in an impressive number of alternative forms: solvates, hydrates, salts, co-crystals, and all these forms can be polymorphic.
These are the reasons why the discovery of a drug, whether destined to treat a new disease or to replace old and less efficacious pharmaceuticals, is undoubtedly a fantastic achievement, but it is also the beginning of a journey in another domain, that of the aggregate form of the new drug, the crystal domain. As a matter of fact, the drug will have to be isolated, its preparation scaled up, purified, stored, packed, distributed, and ultimately administered to patients and all these steps will be carried out, in the vast majority of cases, with the drug in the solid form, sometimes amorphous, most often a polycrystalline material.
To summarize:
i) An API can take many solid forms (polymorphs, solvates, salts, co-crystals) all containing exactly the same active principle but in different structural arrangements and/or associated to different molecules (solvents, coformers, counterions, salts, etc.);
ii) different solid forms will possess different properties, which might affect a drug processing, formulation, distribution, storage, administration to the patient and, ultimately, its therapeutic efficacy;
iii) since there is no way to guarantee that a transformation to a more stable form will occur, or that a more stable form will appear, a preliminary, thorough exploration of the crystal domain is the only way to minimize the chances of the undesired appearance of a new and more stable form at a later stage of development;
iv) besides maximising control on the crystal form of the API, the investigation of hydrates and co-crystals may afford alternative, often improved or innovative properties of the drug;
v) polymorphs, hydrates and co-crystals may meet the requisites of novelty, utility and non-obviousness useful for extending the patenting life of a drug. The quest for new crystal forms of any given API can be both “joy and sorrow” for the academic and industrial researcher.
We have been dealing with crystal forms of APIs for the past thirty years.